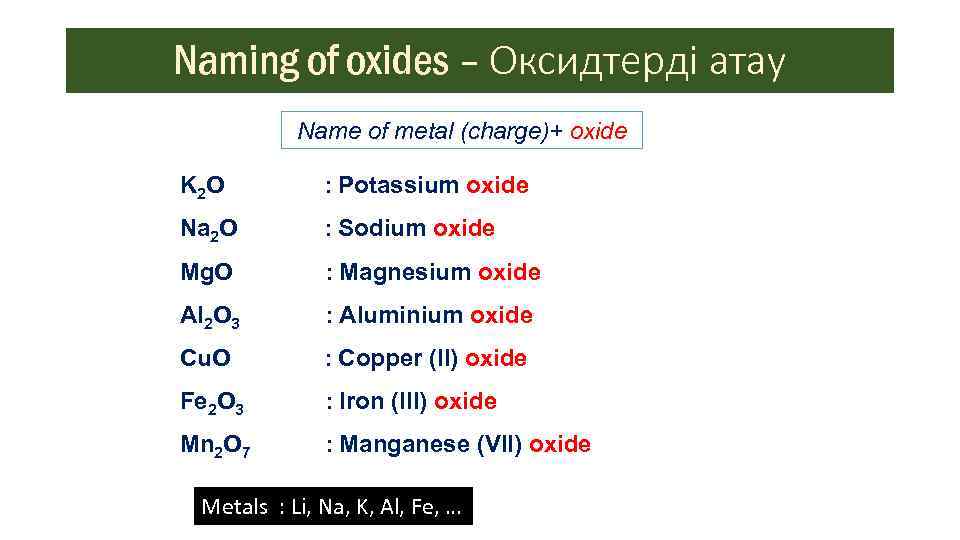
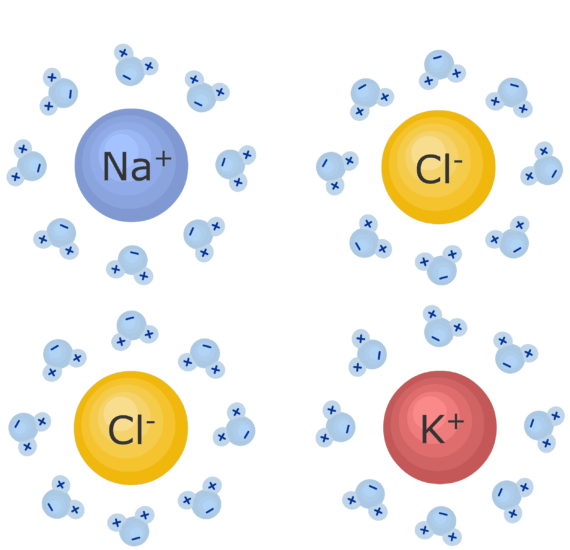
When K And Cl Form An Ionic Bond
When K And Cl Form An Ionic Bond. Ionic bonds involve a cation and an anion. Ionic bonds are formed when a metal atom "loses" and electron to a nonmetal electron that "takes" it, causing the metal atom to become slightly positively An ionic bond formed b/w electropositive element & electronegative element.

When ionic solids melt into liquids though, they do conduct heat and electricity.
An ionic bond is the result of electrostatic attraction between positively bonding occurs most easily when metals react with non-metals.
A covalent bond involves atoms sharing electrons. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. The oppositely-charged ions formed, K+ and Cl-, are then strongly attracted to each other by strong electrostatic forces in the crystal lattice, called ionic bonds Hence, the ionic compound magnesium fluoride with the electron arrangement as shown in above figure is formed.





0 Response to "When K And Cl Form An Ionic Bond"
Posting Komentar